Background Gating
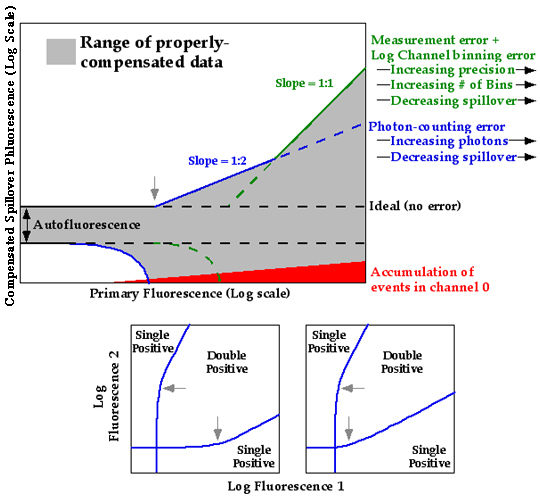
Nearly every flow cytometric experiment requires the discrimination of cells that exhibit fluorescence signal from those that do not. For many experiments, this is relatively straightforward, for example, when the signal is well-separated from negative cells. However, discrimination is not so easy when the signal is low, when the positive population does not separate well from the negative. In addition, in the multicolor world, sensitivity is often highly compromised by the post-compensation spread in autofluorescence distributions (see Compensation An Informal Perspective by Mario Roederer).
An accurate way to identify positive events is the use of an "FMO" control (Fluorescence Minus One). This is a sample that has been stained with every reagent except for the one of interest; the difference between the FMO control and the test sample identifies positive events. In multicolor experiments, compensation-caused spreading in the distribution can be calibrated by using beads singly labeled with each fluorochrome (akin to the process of assessing fluorescence spillover using compensation controls). In FlowJo, the Background Gating Platform uses the beads to compute an event-by-event background for each parameter, providing a way to discriminate positive events from those that look positive because of compensation caused spreading.
Multicolor background gates are best for identifying positive events. The negative populations will always contain a mixture of positive and negative events because of the inescapable overlap in distributions.
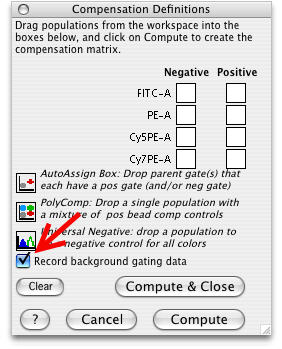
Procedure: In order to calculate the background, you need to compensate your data in FlowJo. Compensation begins by selecting the Platform > Compensate Sample... > Define Matrix menu item. This opens the Compensation Definitions dialog box shown on the right. Click on the Record Background Gating Data button shown on the right with a red arrow. The calculation of background works best with single color compensation beads, but can be done with stained cells.